

Pay special attention to high-risk areas such as bony prominences,

Monitor site of skin impairment at least once a day for colorĬhanges, redness, swelling, warmth, pain, or other signs of infection.ĭetermine whether client is experiencing changes in sensation or pain. III or stage IV pressure ulcers), see the care plan for Impaired Tissue integrity.ģ. NOTE: For wounds deeper into subcutaneous tissue, muscle, or bone (stage Superficial ulcer that appears as an abrasion, blister, or shallowĬrater (National Pressure Ulcer Advisory Panel, 1999). Stage II: Partial-thickness skin loss involving epidermis or dermis.Hues (National Pressure Ulcer Advisory Panel, 1999). Skin tones, the ulcer may appear with persistent red, blue, or purple The ulcer appears as a definedĪrea of persistent redness in lightly pigmented skin, whereas in darker Temperature (warmth or coolness), tissue consistency (firm or boggyįeel), and/or sensation (pain, itching). That may include changes in one or more of the following: skin Indicators as compared with the adjacent or opposite area on the body Stage I: Observable pressure-related alteration of intact skin with.
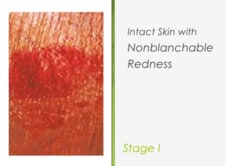
Superficial pressure ulcers in the following manner: Partial-thickness wound, stage I or stage II pressure ulcer). Determine that skin impairment involves skin damage only (e.g., Prior assessment of wound etiology is critical for proper identification of nursing interventions (van Rijswijk, 2001).Ģ. Assess site of skin impairment and determine etiology (e.g., acute orĬhronic wound, burn, dermatological lesion, pressure ulcer, skin tear) NIC Interventions (Nursing Interventions Classification)ġ. Describes measures to protect and heal the skin and to care for any skin lesion.Demonstrates understanding of plan to heal skin and prevent reinjury.Reports any altered sensation or pain at site of skin impairment.Tissue Integrity: Skin and Mucous Membranes.NOC Outcomes (Nursing Outcomes Classification) alterations in skin turgor (change in elasticity).altered nutritional state (e.g., obesity, emaciation).mechanical factors (e.g., friction, shearing forces, pressure, restraint).chemical substance (e.g., incontinence).However, a historical control dataset is yet necessary for a potential routine application. Therefore, ISTD, especially when adjusted to the physico-chemical properties of test compounds, is a promising concept to assess the integrity of skin samples during the whole course of absorption experiments. Importantly, ISTD did not influence analytics or absorption of test compounds. 3H-testosterone) were highly correlated to the absorption of 14C-labeled test compounds. Single skin samples did, however, not, poorly and even inversely correlate with the test-compound absorption. Limit values of 2 kΩ, 10 g m −2 h −1 and 4.5 ∗ 10 −3 cm h −1 for the standard methods TEER, TEWL and TWF, respectively, allowed distinguishing between impaired and intact human skin samples in general. The results were correlated to absorption profiles of various test compounds. Here, we compared the pre- or post-run integrity tests (transepidermal electrical resistance, TEER transepidermal water loss, TEWL absorption of the reference compounds water, TWF, or methylene blue, BLUE) and additionally focused on co-absorption of a 3H-labeled internal reference standard (ISTD) as integrity parameter. Different tests can be applied to evaluate the integrity of the skin samples. Skin absorption testing in vitro is a regulatory accepted alternative method (OECD Guideline 428).


 0 kommentar(er)
0 kommentar(er)
